Expanding traditional boundaries to enhance student learning
My pedagogical approach has several components that focus on developing skills in STEM: (1) data analysis and quantitative skills, (2) data visualization, (3) critiquing the primary scientific literature and media representation of science, (4) communicating scientific outcomes to a broad audience, and (5) thinking creatively and broadly. I am most inspired to help students develop as a scientist, an analyst, and ultimately help them get started on the job market. I seek to help students develop and feel confident about their quantitative and computation skills through course-based research and coding, and I provide a hands-on experience while students become familiar with tools used in STEM.
Science communication is the hallmark of the courses I teach and my research. Despite the highly specialized training and jargon of each scientific field, I truly believe that science is for everyone when we can distill complex data and processes to a form that draws connections. Science communication is a passion that carries through my teaching, research, and outreach.
current courses at ubc
Introduction to Forest Ecology
This course explores the structure and function of forest ecosystems, including: energetics; productivity; nutrient, carbon and water cycling; soils; the physical environment; population and community ecology; disturbance ecology; ecological succession; biological diversity and ecological resilience.
Indigenous research seminar/course series
I am one of the founding members of the Resurgent Indigenous Scholars for the Environment (RISE) Collective at UBC. We were the sole Indigenous tenure-track faculty at the time of our department hiring and we created a space to provide mutual support, co-design research and teaching projects, and be in collective service to Indigenous Peoples within and beyond the university. One of our first accomplishments was receiving funding from the Indigenous Strategic Initiatives Fund at UBC to develop a Science Research Seminar/Course Series that spans our four faculty units. We are designing a new course for undergrads and graduate students which will encompass Indigenous Knowledge and research methodologies. There are very few Indigenous courses in the sciences, so this would fulfill a huge knowledge gap and be available to an enthusiastic group of students. To date, we have held three sessions with Indigenous students, faculty, and staff to get involved in the process, especially through focus groups.
teaching in the time of covid-19 (at smith college)
PLANT PHYSIOLOGY LECTURE
Plant Physiology ballooned to 36 students for spring 2020! How do you move a class like this to remote teaching?! With only two weeks notice, this is my version. Everyone was spread out across multiple times zones with different levels of internet access. Thus, I opted for an asynchronous version of my course with new elements:
Video Modules: My course is typically comprised of 75 minute lectures and is packed with active learning and breakout groups. Honestly, recording my voice while going through the regular powerpoint slides seemed really boring (and this is coming from someone who loves plant physiology). I aimed for topics in video modules as opposed to lectures, with the following features:
Each video module covered only one topic in ~15 minutes (SEE VIDEOS MODULES BELOW).
I used the Whiteboard app from Explain Everything on an iPad Pro (with the Apple Pencil) to record imported presentations. The “stealth mode” for adjusting your recording frame is a lot fun!
The Apple Pencil allowed for dynamic animations, write while recording, highlighters, and drawing graphs for each topic.
The recorded video was then edited with more dynamic features (I import better fonts and adjustable text boxes and images) and merged with other videos in iMovie. I also play around with audio effects.
Each video ends with a cameo from my cat, Chimichanga!
I use the EOS Canon Rebel T7i for recording myself and sometimes I use it for recording other videos (my iPhone was great for these extra videos too). It is worth investing in top-notch equipment that will give high quality videos (nobody wants to see grainy videos of your “bats in the cave” when you record with the laptop at a low level)! If you are working solo, it is incredibly time-consuming to record yourself with this equipment…think running back and forth to adjust lighting, height of tripod, angle, etc!
I use royalty-free music when music is included in my videos.
Each video was posted on YouTube (only the cool kids who took my class had access) and a downloadable mp4 file was posted on Moodle and Slack. Sometimes the mp4 files are too large to be uploaded to Moodle or Slack, which requires software to compress them to a smaller size (I have my favorite, but won’t influence your decision here). Additionally, I posted static slides of the videos.
Study Guides: Typically, I provide guidance and chapter readings for each lecture. Under these circumstances, I wrote a very detailed study guide for each video module with exact pages/paragraphs, summaries, main takeaways, and “what you should be able to do” list. Web links were provided when the book was not adequate.
Slack: I was never a fan of Moodle, but everyone uses it! In addition to Moodle, everything was posted on Slack. Slack was more interactive and it was really helpful for students to see my answers to everyone’s questions.
I adopted a “work at your pace” model and made the remaining exam open book/open notes.
I reduced content for the online version. At first I didn’t like it, but it really made me re-evaluate what I wanted students to takeaway from the course.
Final Project: I gave students the option of working solo or in a group for their final projects. Here are the general aspects to the final project requirements (students were provided with more extensive details on project guidelines):
The Premise: The college is soliciting innovative proposals for answering BIG questions in Plant Physiology. This is for the campus, which may include a new institute/building/program/new method/new equipment/etc. What does the college need?
Give us your pitch in the form of a newsletter. The newsletter format is modeled after research articles from Frontiers in Ecology and the Environment, which are colorful, dynamic, and palatable to the general public.
The pitch must include a BIG research question in Plant Physiology and the rationale.
Whether the project was solo or group based, every single person must be an expert. Example: If I am working solo on a project answering “How can plant uptake of carbon be maximized to help control global warming?”, then I could be a photosynthesis/LI-COR 6400 specialist. If I was in a group, then another expert could be a soil scientist, or hydrologist.
Broader impacts:
Who is this for?
- What program, center, institute, etc. will you establish on campus? (We have AEMES, STRIDE, etc. What other programs are needed?)
Come up with an acronym. Here are some examples:
- Experimental Program to Stimulate Competitive Research (EPSCoR)
- Achieving Excellence in Mathematics, Engineering, and Sciences (AEMES)
- STEM People of Color (STEMPOC, from Mount Holyoke)
Start original hashtags or catch phrases for social media
The newsletter must include a conceptual diagram to support your pitch and some literature cited.
Here are just a few examples of these stellar newsletters! First pages only and names omitted.
VIDEO MODULES
The primary source of reading for the course is Fundamentals of Plant Physiology, 1st edition, by Lincoln Taiz, Eduardo Zeiger, Ian Max Moller, and Angus Murphy. Diagrams used in the video modules are from the following three textbooks:
Video Module 1: The Carbon Reactions
In this basic overview, you will learn about the major inputs and outputs at each phase of the Calvin Cycle. Only key takeaways here (learning a bunch of reactions was not on the priority list). Ultimately, we answer the question: How do molecules of CO2 get processed and create sugar?
Video Module 2: CO2 Response Curves
Hear how plant physiologists use the LI-COR 6400XT to measure photosynthetic capacity. We cover how biochemical limitations prevent plants from continuously increasing photosynthesis in response to increasing CO2 levels.
Video Module 3: Rubisco
Rubisco is a fascinating enzyme, packed with many active sites and can act like a carboxylase (fix CO2) or oxygenase (fix O2). As cool as that is, it can lead to an inefficient process known as photorespiration. Learn how the environment affects when rubisco will influence higher or lower photorespiration.
Video Module 4: C3 versus C4 Photosynthesis
We start by explaining how plants that use the C4 photosynthetic pathway are able to overcome the photorespiration issue found in plants that use the C3 photosynthetic pathway. We finish by comparing CO2 response curves in C3 versus C4 plants.
Video Module 5: Essential Elements
In this brief intro to nutrient uptake, we talk about the fundamentals of essential elements and how nutrient levels affect patterns of plant growth (biomass allocation). In the end, we make draw relationships between growth, age, and leaf gas exchange patterns with different nitrogen levels.
Video Module 6: Symbiotic Relationships
Plants form symbiotic relationships with bacteria and fungi. We learn about the types of plants that form these relationships and the general steps involved in root nodule formation and mycorrhizae associations.
Video Module 7: Biotic Interactions Part I
Plants have a diverse suite of defensive strategies to cope with harmful interactions with other organisms. In this module, we go over the defensive strategies that are always present, which include mechanical barriers and secondary metabolites. However, there are some pathogens and bacteria that have “out smarted” these strategies.
Video Module 8: Biotic Interactions Part II
In Part II of Biotic Interactions, we cover the induced plant defensive strategies when interacting with harmful organisms. We start with cellular responses with defensive compounds. We move on to hypersensitive responses at the local site of infections. Finally, we discuss the basic steps of how plants develop systemic acquired resistance.
Video Module 9: The Wrap Up
In the final module of the semester, we discuss the state of climate change, especially temperature anomalies. Changes in phenology can be linked to temperature anomalies, with big implications for ecosystem health. Plant response to climate change can vary between phenotypic plasticity, acclimation and adaptation, but can plants keep up with accelerating climate change? We end by discussing how plant physiologists must move forward with new experiments and method.
Video module features animations from NASA
PLANT PHYSIOLOGY LAB
The Plant Physiology Lab was an easier transformation than the lecture course. Typically, the lab consists of a three-hour session packed with R & RStudio tutorials and plant measurements (leaf gas exchange, growth, and nutrient content and biomass after harvesting) at the botanic garden greenhouse. Students were already proficient in data wrangling and graphing in R Markdown files prior to moving to the online model. However, they were not able to complete their own data set. I compiled data from previous years that matched the experimental treatments and plant types used during this semester. Here are the new elements in the online model:
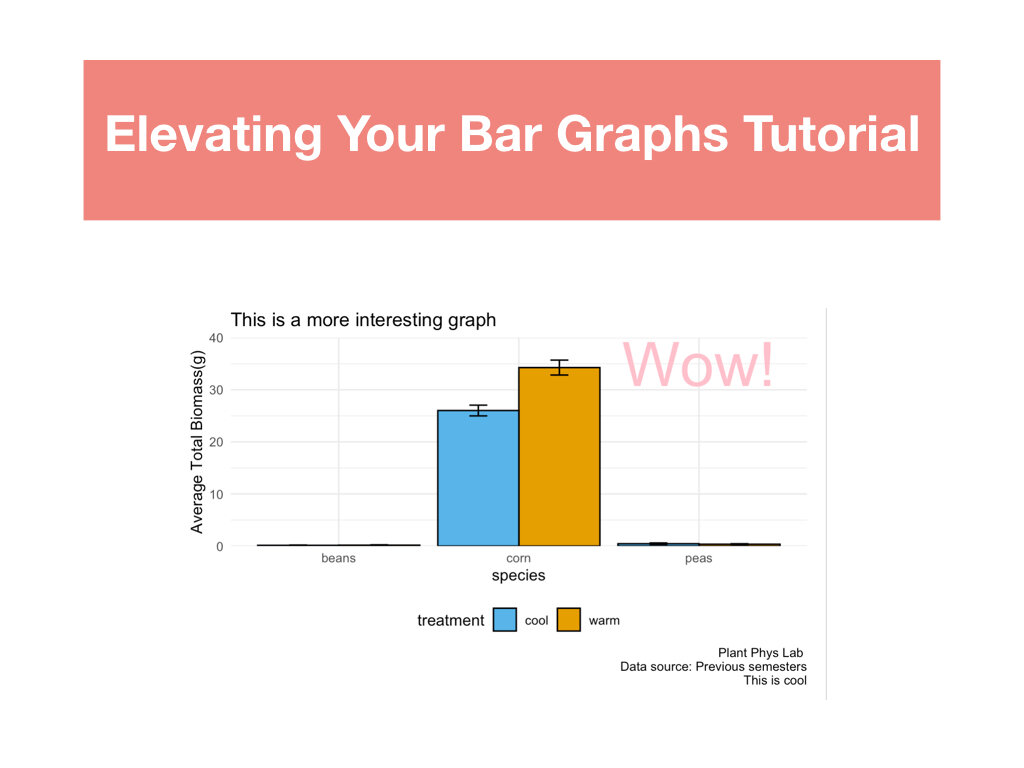
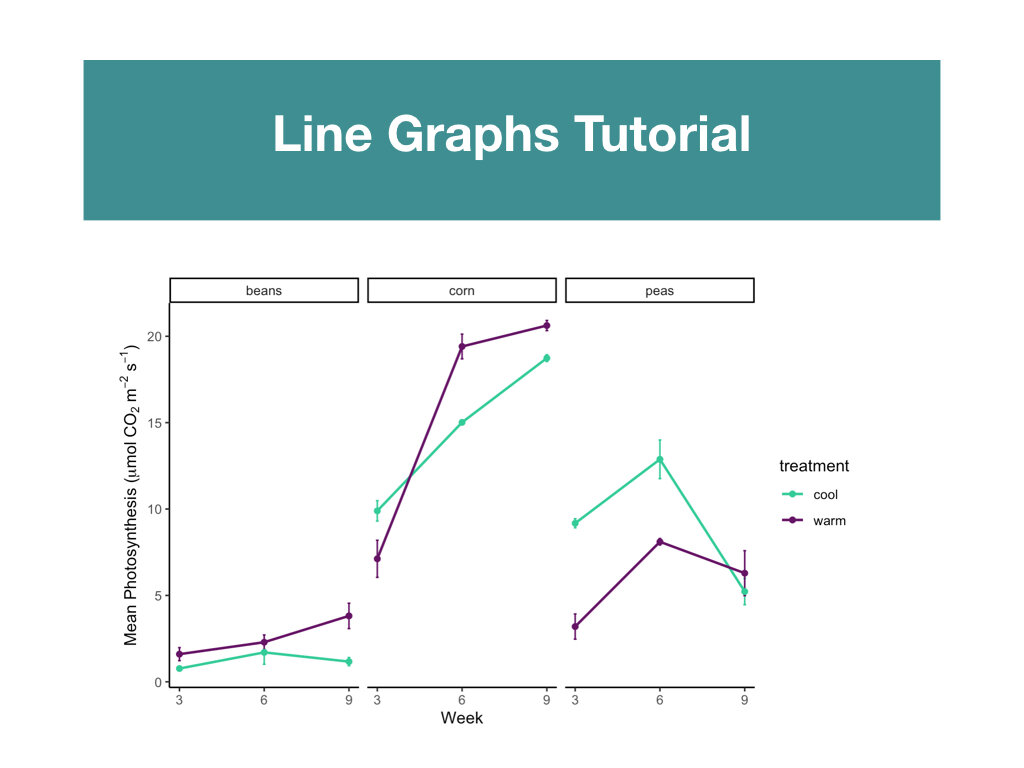
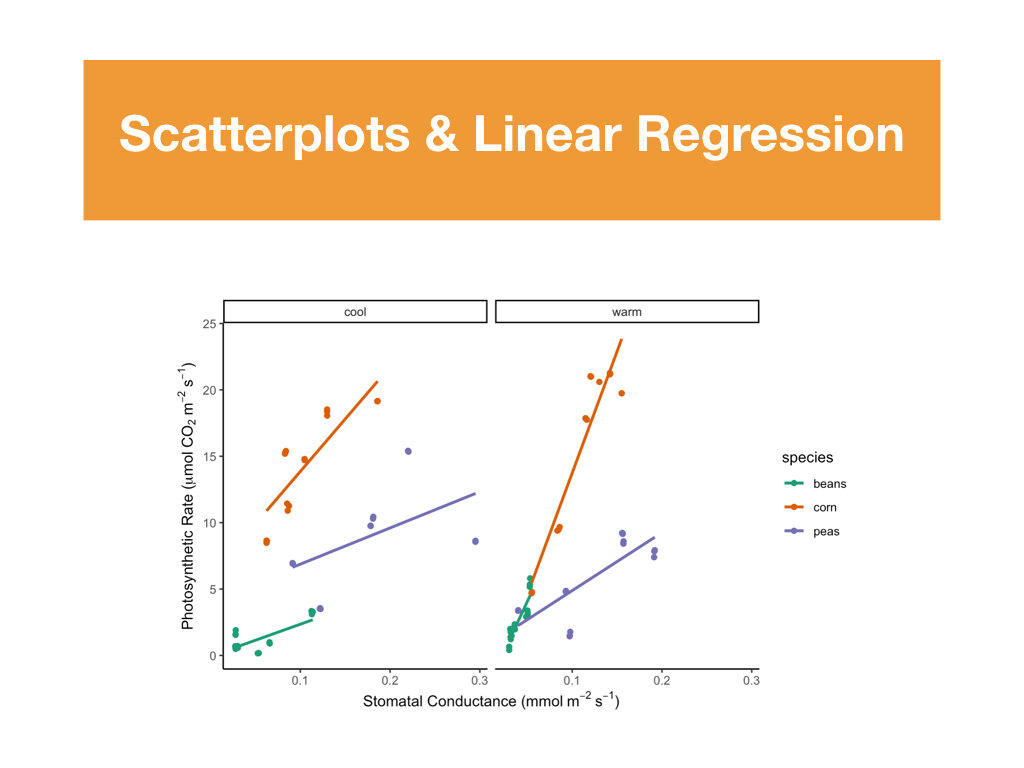
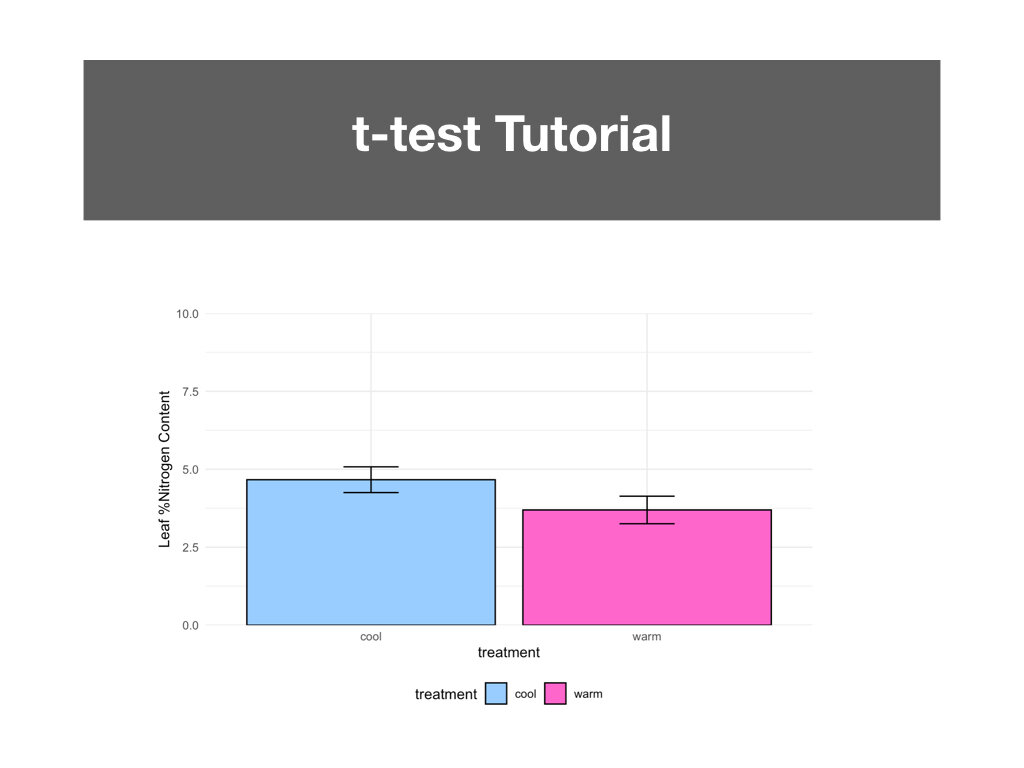
R Tutorials focusing on elevated data analysis and data visualization.
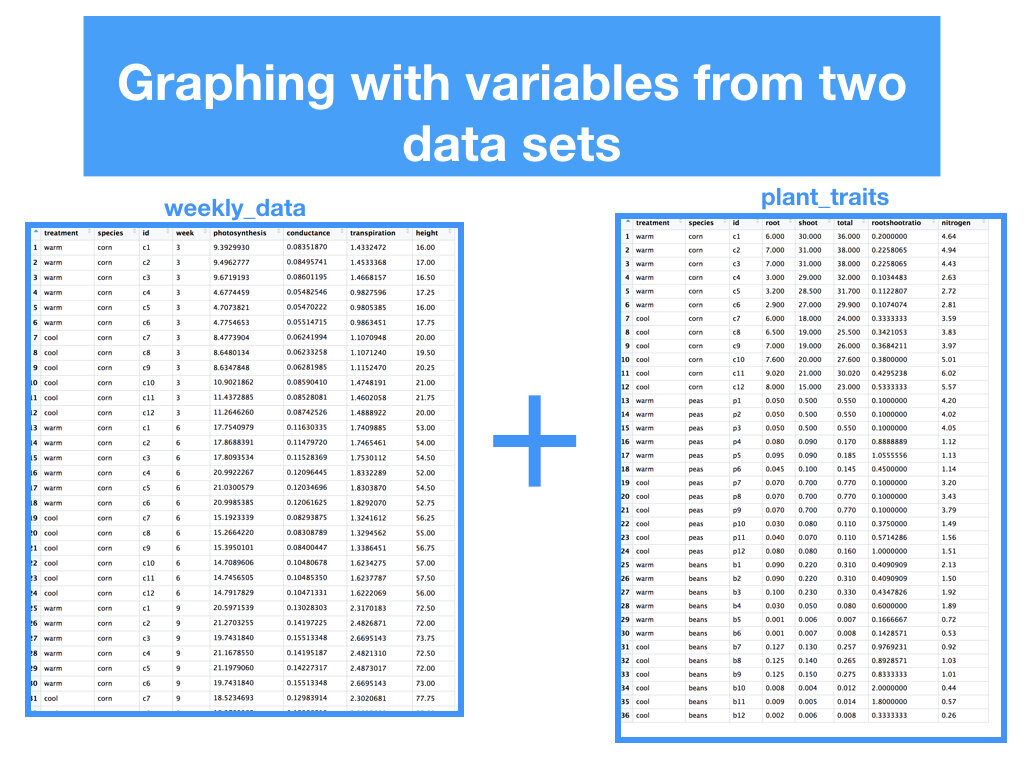
Two data sets that included weekly measurements of leaf gas exchange and growth, and leaf nutrient content and biomass allocation post harvest.
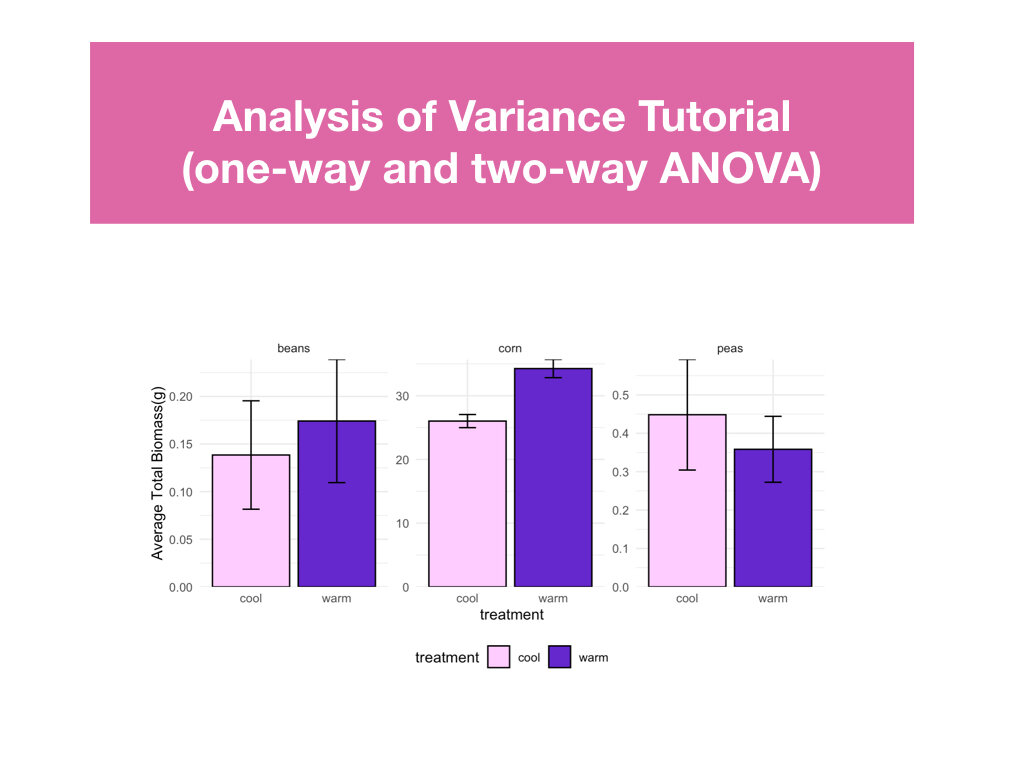
Students made weekly updates to a master R Markdown file complete with their research questions/hypotheses, rationale, summaries of data wrangling and graphing output, and conclusions based on statistical analyses.
Students had to create a conceptual diagram to accompany the conclusions made in their R Markdown files.
Feedback was provided each week and Slack was useful for posting errors in coding with my solutions.
Weekly Tutorials on Data Wrangling and Graphing in RStudio
TEACHING DURING A REGULAR SEMESTER:
Watch this video to learn more about my academic background and how it guides my approach to teaching:
BIO 206: Plant Physiology Lecture
This course is designed as an introduction to Plant Physiology. Students will learn fundamental concepts and innovative research that encompasses this exciting field based on the functioning of plants. Through this course students will learn about the biochemical and molecular processes to whole- plant function, such as germination, growth & development, phenology, plant diseases & defenses, and adaptations to stress. We will take an eco-physiological approach for using the key concepts in plant physiology to understand dynamic ecosystem processes, response and adaptation to climate change, and biodiversity of plant communities.
BIO 207: Plant Physiology Lab
This lab focuses on the impacts of the environment on plant physiology. Through course-based research projects, students learn key concepts in plant physiology in order to understand plant function. Students choose grow their own plants from seeds and expose them to various treatments at the Smith College Botanic Garden. Students take various measurements including leaf-level gas exchange (photosynthesis) using sophisticated equipment, growth, nutrient allocation, and water-use efficiency. Students use RStudio for data analysis and data visualization. By the end of this course, students gain valuable experience in experimental design, use state of the art equipment, learn how to statistically analyze their data, and become proficient at coding in R.
BIO 355: ecoPhysiology Lecture
Ecophysiology is based on the interaction between an organism’s physiology and its environment. Topics in this course include the impacts of changes in climate and resources on physiological processes, with a strong emphasis on plants. The role of microbes and animals are also discussed through a food-web approach to understanding ecosystem patterns and processes. Through understanding the physiology of the cells and individuals, students will be able to understand how to scale up to the physiology of the ecosystem.
BIO 356: ecoPhysiology Lab
Students explore the creative and artistic side of science through independent research projects that address world-pressing problems in Ecophysiology. Projects will be based on large, long-term, publicly available datasets from world-renowned field stations. Students will use RStudio to become proficient in the art of data visualization, data exploration, and data analysis. We explore how to make scientific presentations come alive and make research more palatable to the general public. The course ends with a scientific poster symposium.
BIO 390 Seminar: Ecological impacts of Global Change
I have designed a course that incorporates (1) a critical analysis of the current global change research and how it is represented by the media sources and (2) exercises and projects that help students conceptualize the most up-to-date global change research and present a public friendly version of the outcomes. The course will primarily consist of student led discussion sessions on recent scientific developments and blogs. The semester culminates with the development of a newsletter that incorporates student creativity and research. Ultimately, the course addresses pressing global change problems and investigates the role for adaptation and mitigation. Students will be able to enhance their public speaking and writing skills while exploring the vast knowledge of research focusing on the impacts of global change.
BIO 132 (formerly known as BIO 150): Cells, Physiology, and Development
Students in this course investigate the structure, function and physiology of cells, the properties of biological molecules, information transfer from the level of DNA to cell-cell communication, and cellular energy generation and transfer. The development of multicellular organisms and the physiology of selected organ systems will also be explored. In addition to lectures, each student will participate in discussion sections that focus on data analysis and interpretation while integrating mechanisms across scales.